ABOUT
ClicBio is revolutionizing the treatment of metabolic diseases by targeting the hunger pathway with a groundbreaking mechanism that acts as a pivotal molecular switch, regulating appetite through the modulation of hypothalamic neurons. In preclinical obesity models, inhibiting CLIC1 has been shown to induce weight loss comparable to GLP-1 agonists, while operating through an independent and additive pathway. Research further highlights the additional benefits of CLIC1 inhibition beyond appetite regulation, including increased energy expenditure, a potential reduction in gastrointestinal side effects commonly associated with GLP-1 therapy, and broad anti-inflammatory action. This innovative approach leverages a single small molecule to target multiple aspects of metabolic disease. ClicBio is advancing a portfolio of oral, small-molecule CLIC1 inhibitors toward human clinical testing, paving the way for a new era of hunger-modulating therapeutics that can work alongside or beyond GLP-1 treatments to more effectively combat obesity and metabolic disease.
LEADERSHIP & DEVELOPMENT TEAM

John Dobak, M.D.
CEO and Co-Founder
Executive Chair, Leios Therapeutics
CEO, DermTech (NSDQ: DMTK)

Jay Hagan
Board Member and Co-Founder
CEO, Regulus Therapeutics (NSDQ:RGLS)
Former COO, Orexigen
Managing Director, Amgen Ventures

Bruce Steel
Board Member and Co-Founder
CEO Equillium (NSDQ:EQ)
Former Managing Director, Biomed Ventures

Olivia Osborn, Ph.D.
Co-Founder/Advisor Metabolism and Obesity

Gregg Timony
SVP, DMPK and Toxicology
Escient, Receptos

Adam Yeager, Ph.D.
VP, Chemistry
Escient, Receptos

Andy Jennings, Ph.D.
Advisor
Structural Biologist/Computational Chemistry
Scientific Advisors

Paul Reider, Ph.D.
Merck

Hugh Rosen, M.D., Ph.D.
Scripps Research Institute

Louis Aronne, M.D.
Weill Cornell Medicine
Our Science
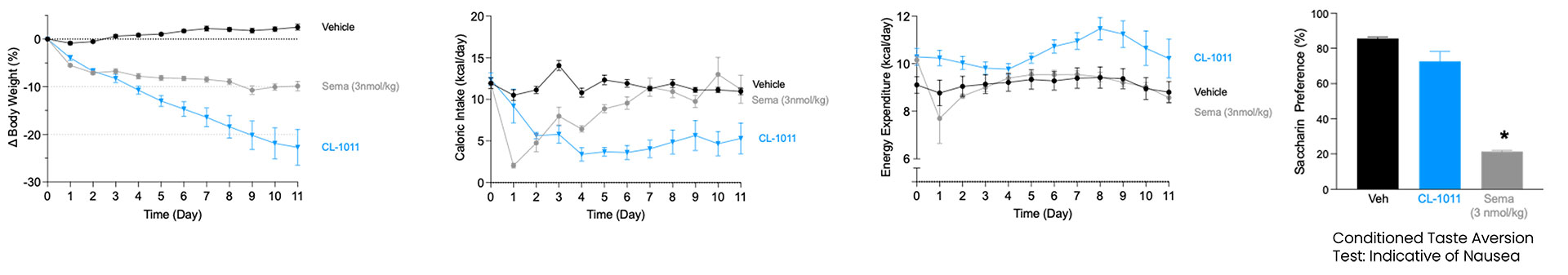
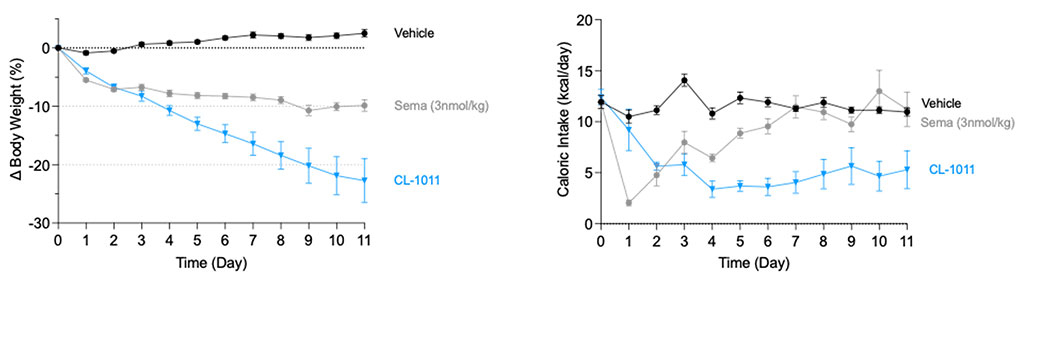
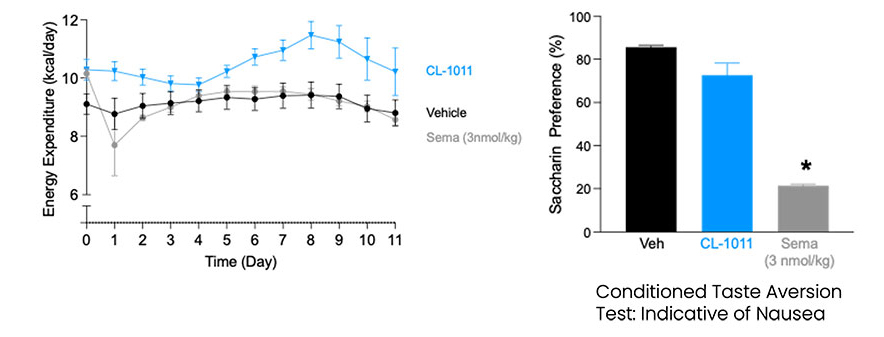
CLIC1 was identified as a key regulator of energy balance through gene-expression profiling of the hypothalamus following weight gain. Subsequent studies across multiple preclinical models—each highly predictive of human outcomes—confirmed CLIC1’s central role in controlling food intake. Inhibition of CLIC1 produces weight-loss effects comparable to those achieved with GLP-1 agonists, and when combined, the two mechanisms act additively to deliver enhanced efficacy.
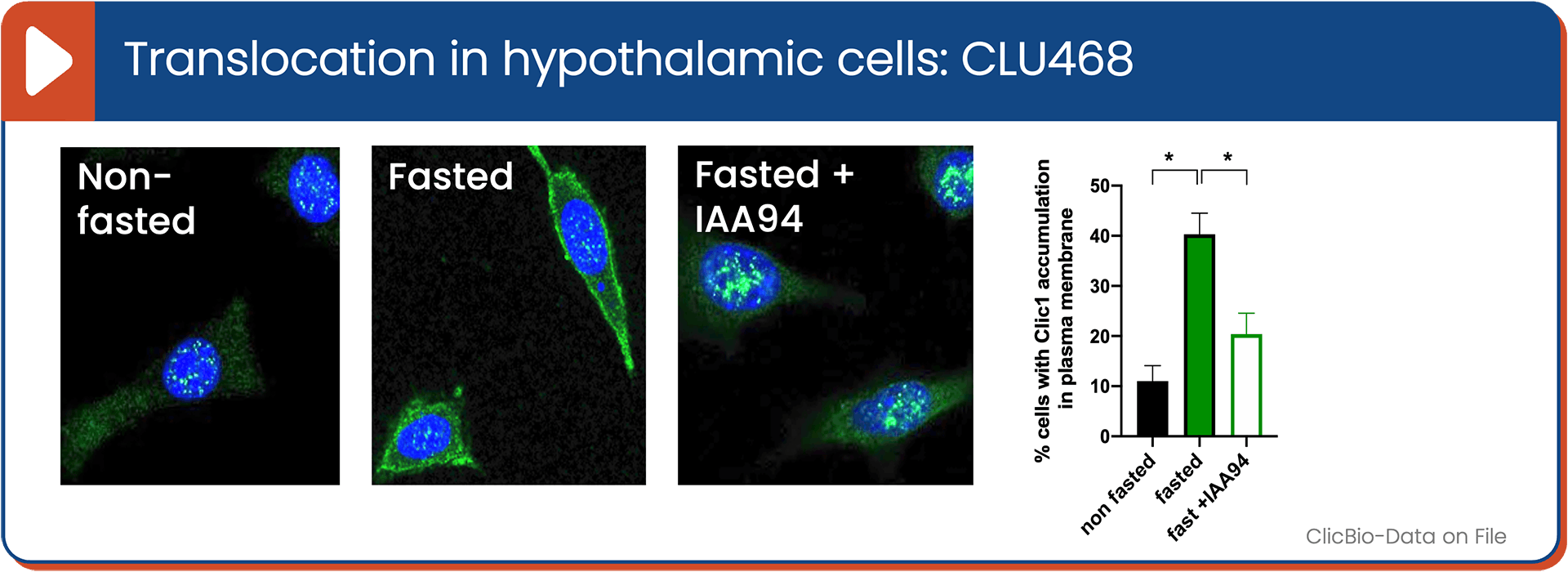
Fasting-Induced Translocation of CLIC1 to the Membrane
CLIC1 is a unique, dimorphic protein that exists in both a soluble cytosolic form and a membrane-bound chloride ion channel form. Within the hypothalamus—the brain’s center for hunger and satiety—CLIC1 is highly enriched in AgRP neurons, the key drivers of feeding behavior. Under fasting conditions, CLIC1 translocates from the cytosol to the neuronal membrane, where it becomes an active ion channel. We believe this shift increases AgRP neuron excitability, promoting hunger and food intake. Therapeutically blocking CLIC1’s membrane translocation reduces neuronal activity in these hunger circuits, resulting in decreased food intake, weight loss, and improved insulin sensitivity.
CONNECT
Thank you for your interest in ClicBio. Complete the form below and we will get back to you soon.